For more than fifty years, the federal government has maintained that cannabis is a Schedule I drug, meaning that it has a high potential for abuse and no accepted medical value. That changed last week (somewhat) when the Department of Health and Human Services (HHS) recommended to the Drug Enforcement Administration (DEA) that cannabis be placed in Schedule III, meaning that it has moderate to low abuse potential, a currently accepted medical use, and a low potential for psychological dependence.
Why now?
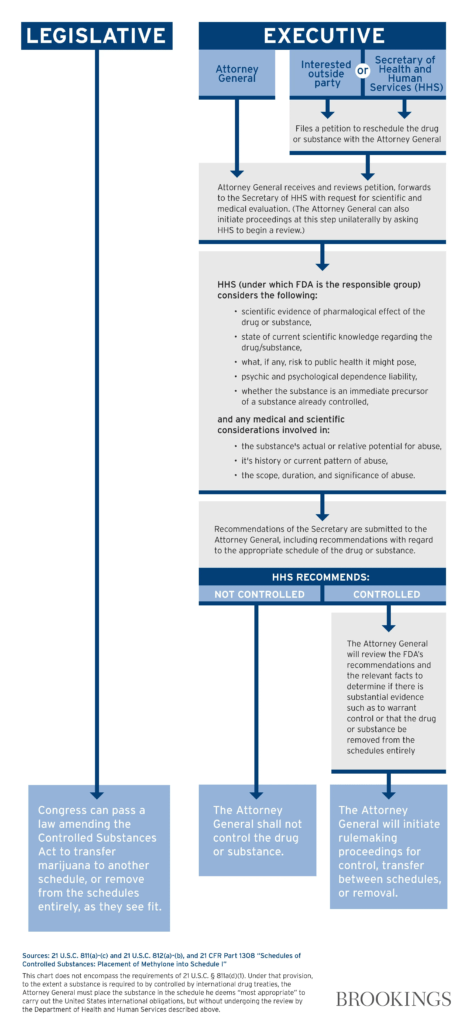
In October 2022, the Biden Administration announced that it would ask the Secretary of HHS and the Attorney General to initiate the administrative process to review expeditiously how marijuana is scheduled under federal law. As the Brookings Institute outlined years ago, the Executive process for rescheduling is much more complex than the Legislative path.
It’s no secret that the presidential election is barely more than a year away, and the President seems to be looking to make good on his campaign promise to reform the nation’s marijuana laws.
What does this mean?
First off, it’s critical to note that HHS’ recommendation to DEA is just that: a recommendation. It is non-binding. The DEA may come to the same conclusion that HHS did, but is not required to.
If cannabis is moved to Schedule III of the Controlled Substances Act, one positive outcome would be that 280E would no longer apply to plant-touching businesses, removing an incredibly punitive and debilitating provision in the tax code.
According to NCIA’s board chair emeritus, Khurshid Khoja, Esq., “…it’s important to remember that rescheduling would not apply the federal Food Drug and Cosmetic Act (FDCA) to marijuana for the first time—it applies right now, and like the federal Controlled Substances Act (CSA), would continue to apply after rescheduling. But absent any statutory authority permitting FDA to do otherwise, the FDCA would continue to apply after descheduling too, just as it does to hemp products.”
Others claim that the shift to Schedule III would have minimal impact on businesses and individuals. Here at NCIA, we’re cautiously optimistic but recognize that moving cannabis to schedule III could have some limited benefit but does nothing to align federal law with the 38 U.S. states which have already effectively regulated cannabis for medical or adult use.
What now?
Now that HHS has made their recommendation, the DEA will begin its scheduling review process.
Many are divided about what a move to Schedule III would actually look like. Yes, there would be the elimination of 280E, but what about enforcement priorities? Interstate commerce? Criminal penalties? There are so many unknowns.
NCIA has previously produced a common sense, workable roadmap for that federal comprehensive reform and provided detailed feedback on legislative efforts. It is time for Congress to follow the will of the American people. Don’t get me wrong, there’s no doubt that this recommendation is a step in the right direction and is long overdue. But we can’t lose sight of the ultimate goal: removing cannabis from the Controlled Substances Act entirely.
Have questions?
Join NCIA on September 14 at 1 pm ET for an engaging webinar where we will unpack all your questions! Register today and don’t miss your chance to hear more about what this means for the cannabis industry and your business.




Follow NCIA
Newsletter
Facebook
Twitter
LinkedIn
Instagram
–